Corals, wound detection, and theoretical physics
Exploring how Loughborough University academics are looking to tackle major issues related to biofilms
Biofilms are central to our most important global challenges, from antimicrobial resistance and food safety to water security, and they have a significant economic, social and environmental impact.
But ask a person on the street to explain what they are, and you’ll likely get shrugged shoulders.
To help the general public understand what biofilms are and why they are so important, the National Biofilms Innovation Centre (NBIC) – which Loughborough University is a research partner of – has launched the #BiofilmAware campaign.
To coincide with the initiative, VOLUME has taken a closer look at three of Loughborough's biofilm projects that aim to advance this important field of work.
But first, let us explore what biofilms are and why we should study them.
What is a biofilm?
A biofilm is formed when numerous living microorganisms, such as bacteria or fungi, group together and then evolve and grow as a collective.
Without realising it, you actually see biofilms everyday – examples include dental plaque, slime on rocks in streams and pond scum.
Biofilms grow particularly well in wet areas, and the light pink areas in your shower, or the grey parts you can see in pipes at home, are also biofilms.
Biofilms are often detrimental, with biofilm infections estimated to be responsible for up to 80% of all infections in humans and animals.
However, beneficial applications in which biofilms can be exploited also exist – for example, ‘Bioremediation’, a cost-effective eco-friendly process of degrading harmful pollutants from the air, soil and water into non-toxic substances using microorganisms.
Gaining a greater understanding of the composition of biofilms and how to prevent, detect, manage and engineer them, would present benefits across a range of sectors, particularly in the healthcare industry where managing infections effectively is a growing issue globally.
So, what is Loughborough University doing?
From the Department of Mathematical Sciences to the Department of Chemistry to the School of Mechanical, Electrical and Manufacturing Engineering’s Centre for Biological Engineering and beyond, important research into biofilms is being conducted across the Loughborough University campus.
Below, Dr Marco Mazza, Professor Andrei Malkov, and Dr Sourav Ghosh, give an overview of their work and upcoming research papers, with topics ranging from tackling tuberculosis using a chemical found in corals to helping the healthcare industry by developing a rapid wound detection test.
From free-floating microorganisms to biofilm
Understanding an important transition that could help tackle medical implant infections

The ability to attach and implant devices in the human body is crucial in modern medicine but a big problem with their use is that they provide a surface for bacteria to colonize on and turn into infectious biofilms.
Biofilm-related infections are among the top four leading causes of death in hospitals so everything researchers can learn about the early phases of a biofilm could become a tool to eradicate them.
Dr Marco Mazza, Lecturer in Applied Mathematics, is the group leader of ‘Nonequilibrium Soft Matter’ – a research group dedicated mainly to theoretical physics.
Through collaborations with experimentalists, the group are trying to understand the early phase in the life of a biofilm, that is, the transition from the very beginning, when there is no biofilm and simply some microbial cells are swimming in a fluid, to the phase when the cells find a surface to colonise, and start reproducing on it.
One of the team’s notable successes is that they discovered a way to monitor biofilm composition and growth while the biofilm forms (paper here) – this is important as it may provide a means for medical professionals to test the response of a biofilm to different treatments that aim to prevent infection.
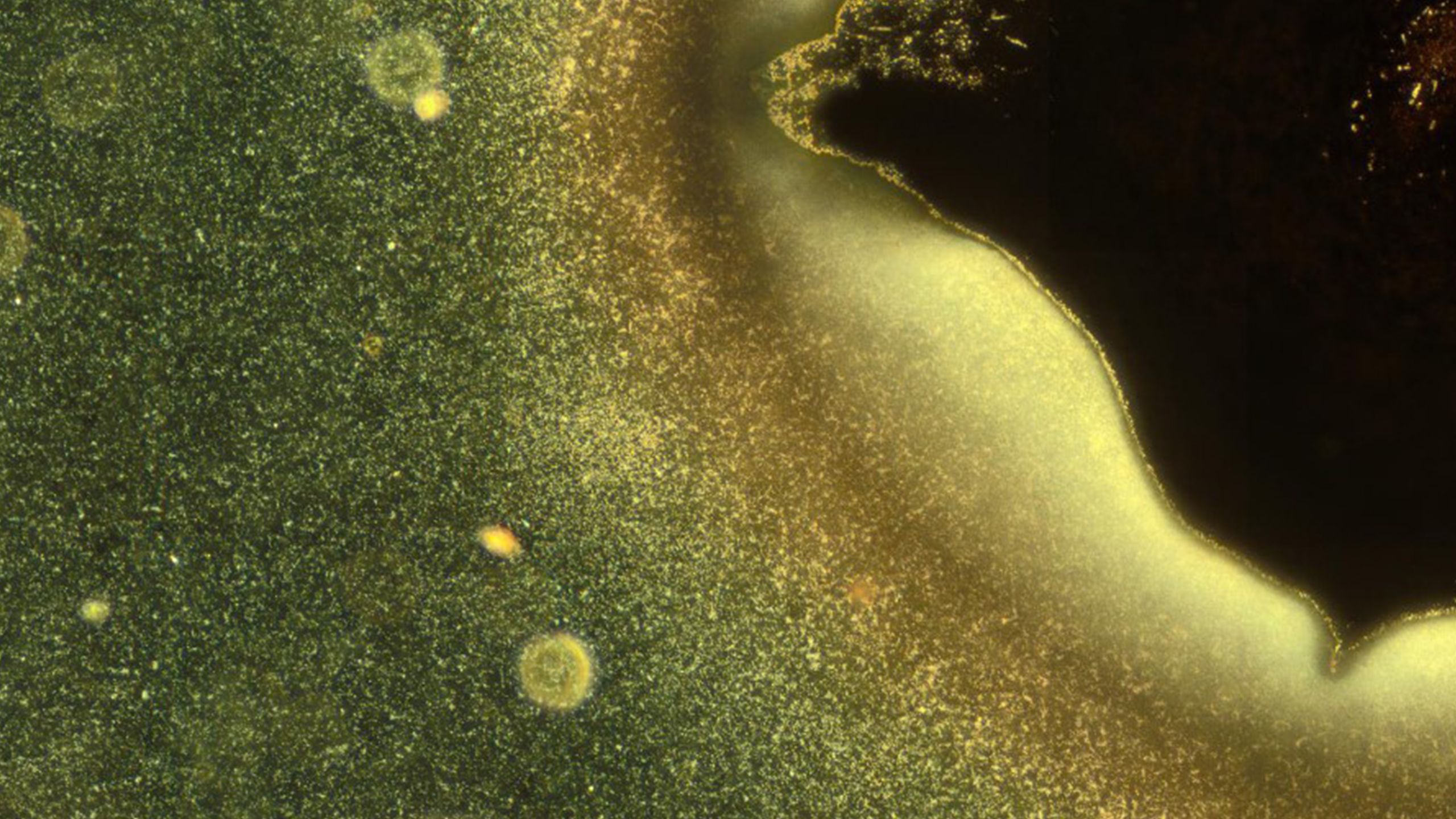
Figure caption: Most microorganisms do not float individually in water but live together in organised communities called biofilms. In this microscopy image, some bacteria (bright particles) are shown as they take the first steps in the formation of a biofilm. Image credits: G. Schkolnik & M. G. Mazza.
Figure caption: Most microorganisms do not float individually in water but live together in organised communities called biofilms. In this microscopy image, some bacteria (bright particles) are shown as they take the first steps in the formation of a biofilm. Image credits: G. Schkolnik & M. G. Mazza.
The researchers are currently exploring different bacterial species and their specific strategies of motion and surface colonisation.
The team’s research has potential to aid in the development of medical applications to treat bacterial infections.
Of the research’s importance, Dr Mazza said:
“Microbial life is fundamental for the ecosystem – more complex life forms in soil and water depend on the activity of microbes – and for the human body – our guts contain enormous numbers of microorganisms necessary to protect us and even to produce vitamins. Understanding how pathogens operate can provide additional weapons to fight them."
Tackling tuberculosis
Exploring if chemicals found in corals can prevent the formation of drug-resistance cells and biofilms that make the disease hard to treat

Biofilms are an integral part of organic chemistry expert Professor Andrei Malkov’s research programme focused on tuberculosis (TB) infections.
TB is a global health problem and, according to the World Health Organisation, it took the lives of 1.4 million people in 2019 and a further estimated 10 million people fell ill with the infectious disease last year, which mainly affects the lungs.
There are different forms of TB, some of which are treatable and curable.
However, ‘multidrug-resistant tuberculosis’ (MDR-TB) is a form of TB caused by bacteria that does not respond to the most effective first-line anti-TB drugs and in severe cases, it also does not respond to the most effective second-line drugs, meaning it remains a public health crisis and a health security threat.
Mycobacterium tuberculosis (M. tb) – the pathogenic bacteria that causes TB – can develop this resistance to antibiotic therapy by producing drug-tolerant cells known as ‘persister cells’.
M. tb also has the ability to form biofilms inside the lungs, as well as on the surfaces of medical devices, that are abundant with these persister cells.
Bacterial persistence coupled with biofilm formation is directly associated with failure of antibiotic treatment of TB, therefore it is important to continue search for new drugs targeting formation of persister cells.
Professor Malkov is working with biologists and chemists to find new, small molecules capable of inhibiting persister cell and biofilm formation in the hope it will lead to more effective antitubercular therapies.
He told VOLUME one of the team’s recent successes is the identification and synthesis of chemical compounds found in a species of soft coral that, during ‘in vitro’ experiments (commonly referred to as ‘test tube experiments’), exhibited the ability to inhibit persister cell formation and eradicate biofilms formed by M. tb pathogens.
The team, led by the Russian Academy of Sciences, have submitted a paper on the topic and their findings to the journal Cell Chemical Biology and are currently revising the manuscript according to reviewers’ comments.
Professor Malkov commented:
“We have identified a small molecule that inhibited formation of biofilms in the laboratory settings. The investigation is now focusing on establishing the exact mechanism of action of the new compounds and making further modifications to the compound structure to improve its activity.”
He continued:
“The identified bioactive natural product analogue may serve as a promising new lead compound for developing novel antitubercular therapies relying on the suppression of persister cell formation and thus effective against chronic and recalcitrant tuberculosis infections.”
Helping wounds heal
Developing a rapid test to detect harmful biofilm-forming bacteria that cause chronic and non-healing wounds

Chronic or non-healing wounds, such as in people suffering from diabetes or burns, is a severe healthcare problem.
For diabetes alone, among the 55 million people in Europe diagnosed with the condition, the lifetime risk of developing a chronic wound, referred to as ‘diabetic foot ulcer’ (DFU), is as high as 25%. In the UK, the NHS costs to due DFU are £10 billion per year and growing.
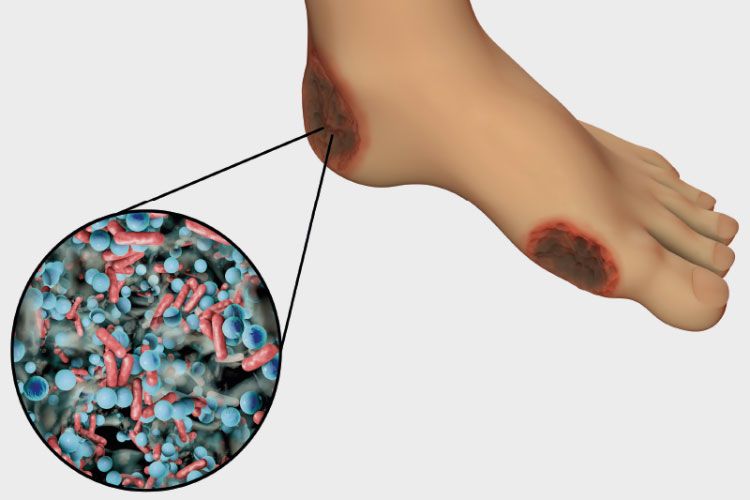
Image: Diabetic foot ulcer
Image: Diabetic foot ulcer
Interpreting whether a wound is going to heal or lead to a diseased state is a complex problem and as a result it is hard to determine the best course of treatment.
Biofilms are the fundamental reason that chronic wounds do not heal in a timely manner and they form when microorganisms – most commonly bacteria - adhere themselves to the wound surface.
However, bacteria can colonise on wounds without causing an infection, so developing a test to simply get a ‘yes/no’ answer for the presence of bacteria is not massively beneficial.
Dr Sourav Ghosh, a healthcare engineering expert, and Praveen Kumar Kaveri, a PhD student in his team of researchers, are developing a rapid, simple, single-step fluorescence-based test that aims to provide crucial information on wound pathogenesis, i.e. whether a wound will heal easily or become non-healing.
The test aims to detect wound infection by determining the number and type of bacteria in a biofilm – known as the ‘bacterial load’ – and then determine the virulence of the bacteria [its ability to prevent wound healing and cause damage to the host].
Dr Ghosh says the research team have had promising results so far and have been able to rapidly detect bacteria quantitatively from a wound mimic sample and confirm at the same time if it is a gram-negative or a gram-positive species, which would help the clinicians in better treatment selection.
In addition, the team have had successes in quickly determining the efficacy of different antimicrobial dressings in killing bacteria.
A paper on the test and the team’s results is expected to be published in early 2021.
Dr Ghosh plans to build on the research by moving to clinical wound samples and developing the capability of detection of gram-specific bacterial load and common virulence activity markers from these samples.
“Our research could potentially lead to the creation of the first rapid near-patient test for holistic assessment of a wound – bacterial load and infection state – allowing timely and evidence-based optimal management, saving significant costs.
“Such a test would also allow the majority of wound care and management load to be transferred from a more expensive hospital-based setting to home and primary care settings.
“Besides reducing the healthcare load, this will also allow us as a society to take better care of the frail and the elderly, particularly during new-age pandemic situations.”
To be kept up-to-date on research conducted at Loughborough University, follow @LboroUniPR on Twitter, an account that shares news stories on the latest papers and findings from campus academics.
For more on the NBIC campaign, click here.
Photo credits
- Several photos were supplied by the National Biofilms Innovation Centre
- Unsplash
- Getty Images
Share this page

